Spotlight on
HTM 02-01 PRODUCTS

products

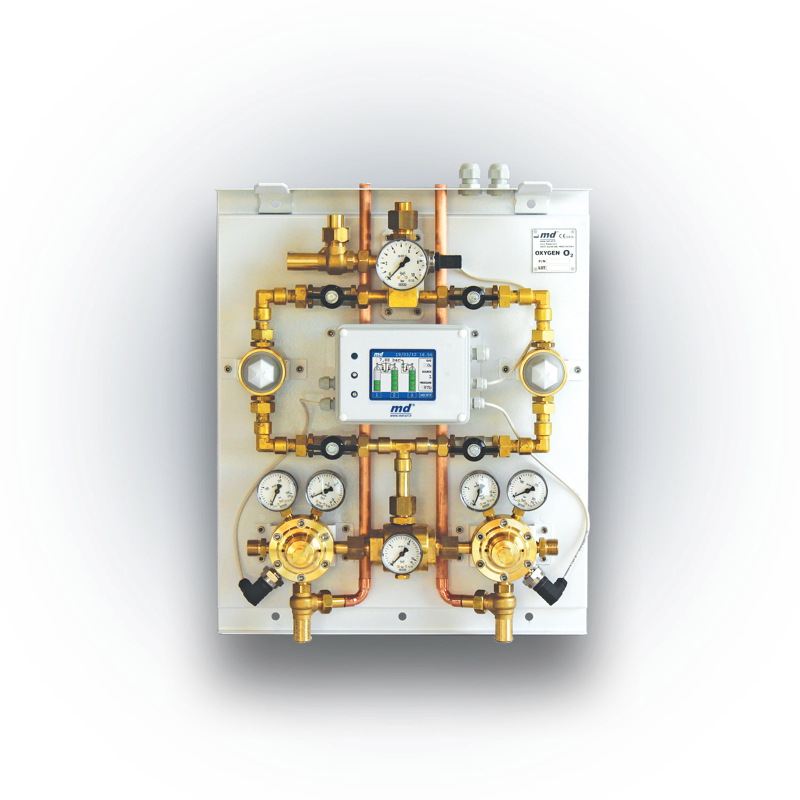
Medical gas equipment and accessories
MD is the leader of the Italian market for the manufacture of medical gas equipment. Read more
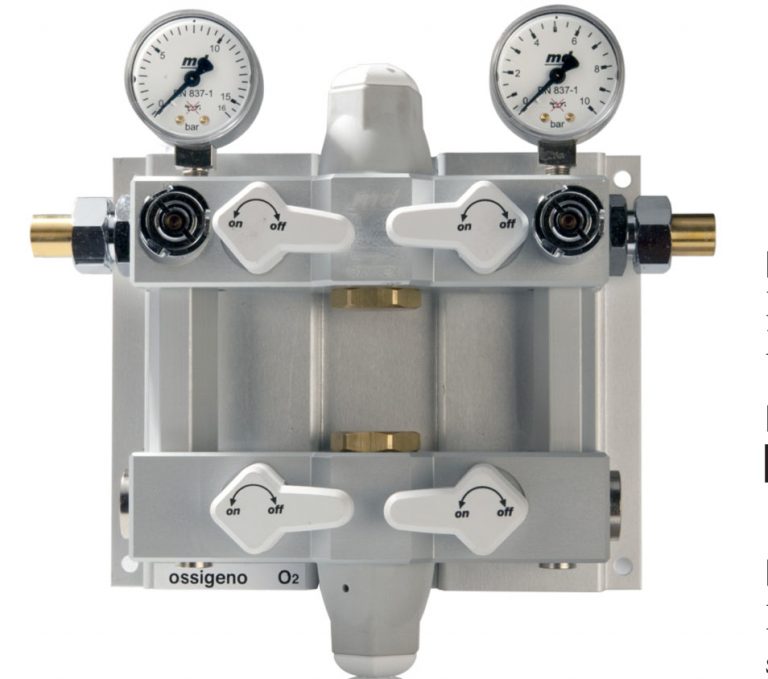
CE marked area valves
Our CE marked area valves are 100% Made in Italy. A sturdy product to ensure quality and reliablity to our customers. Read more